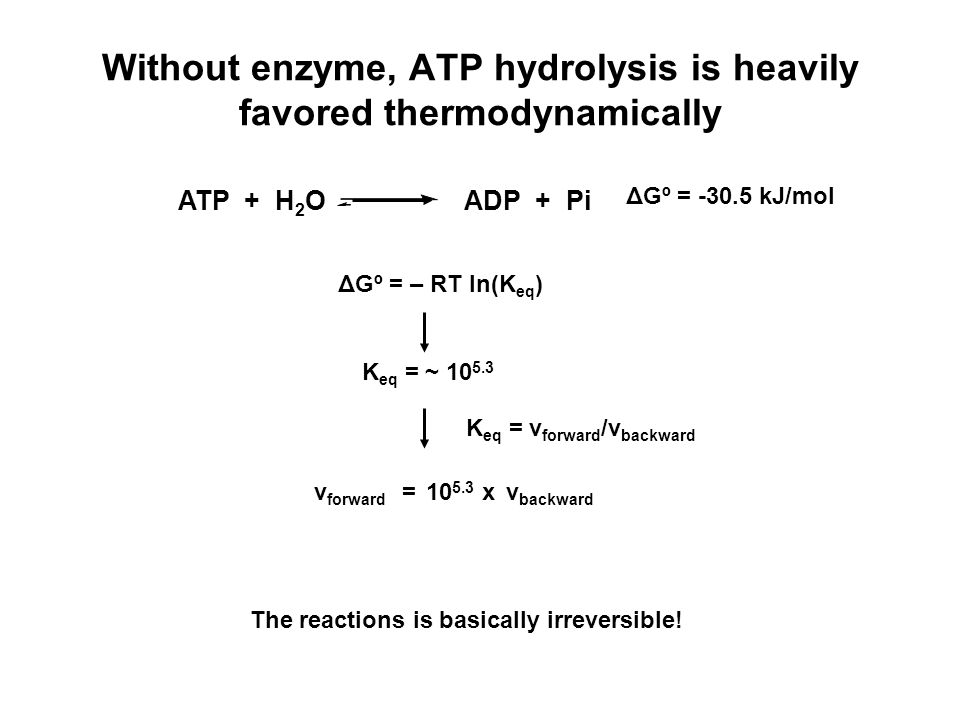
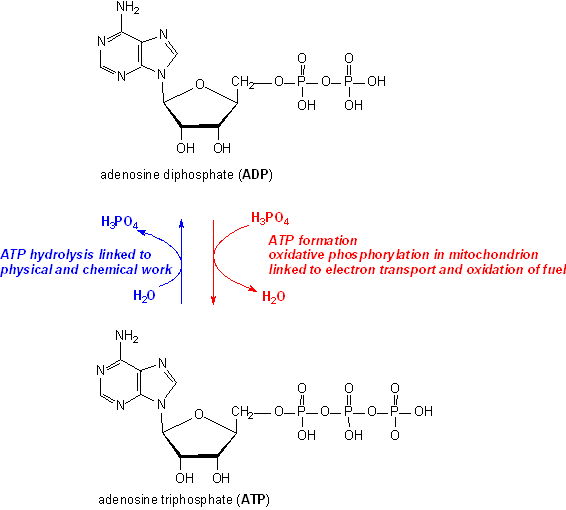
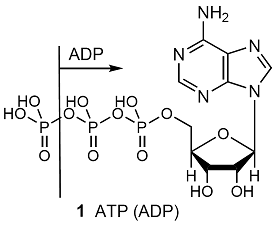
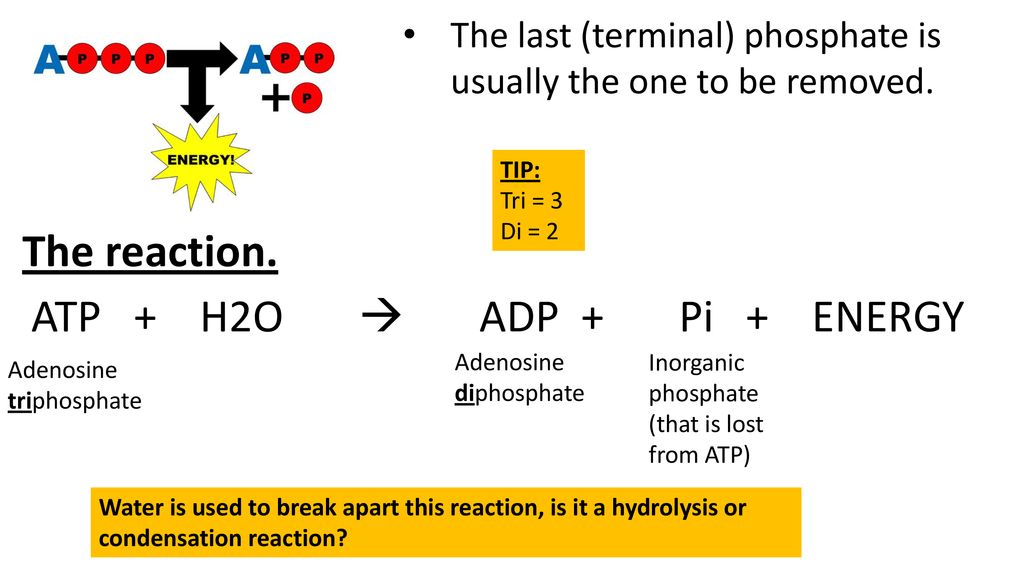
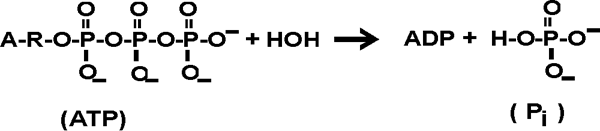SOLVED: ATP +H2O ADP + Pi ∆G°' = 30.5 kJ mol-1 The final ∆G°' for the coupled reaction is –30.5 kJ mol-1. The reaction this is coupled is: A + C B

A water molecule is added to an atp molecule to break atp down into adp and a phosphate group. write the - Brainly.com
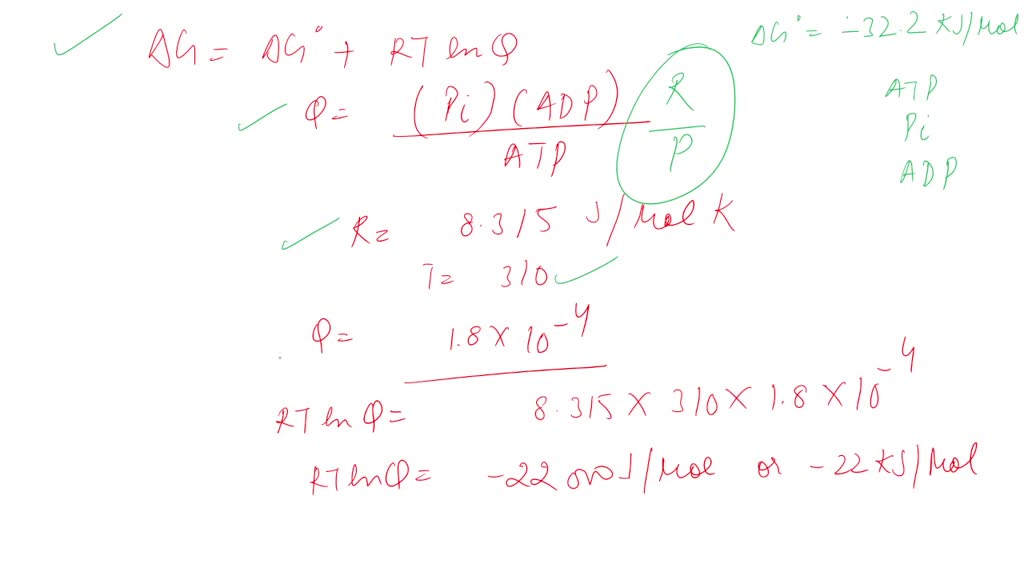



.PNG)
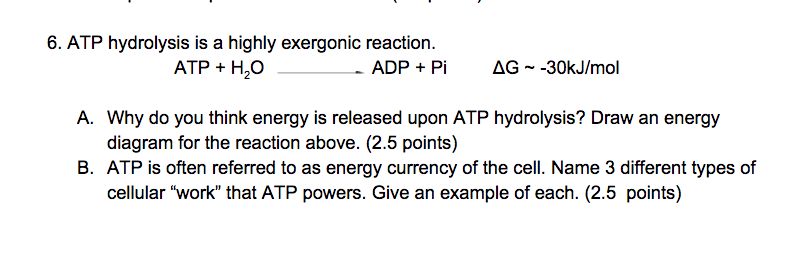
![Solved ATP + H2O → ADP + Pi [AGO' = -7.3 kcal/mol ] Question | Chegg.com Solved ATP + H2O → ADP + Pi [AGO' = -7.3 kcal/mol ] Question | Chegg.com](https://media.cheggcdn.com/media/c65/c65cc5ef-e21f-4e67-8f65-9edf4841d0ef/phpTtmcCV)
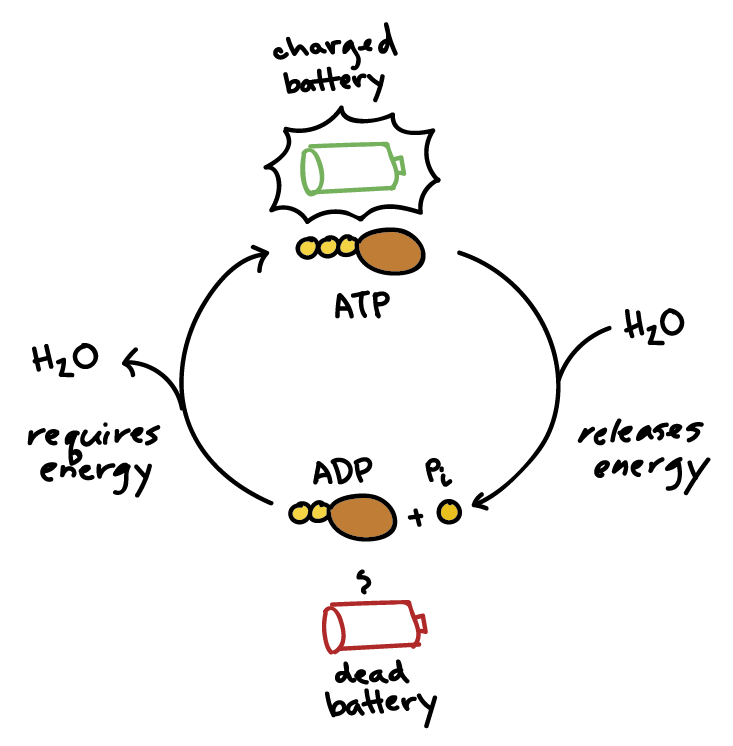
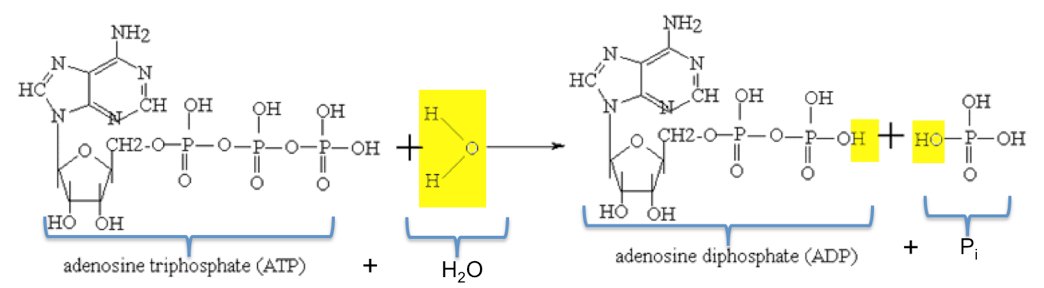
![Energy in breaking bonds [ATP and ADP]? | ResearchGate Energy in breaking bonds [ATP and ADP]? | ResearchGate](https://www.researchgate.net/profile/Yufei_Huang20/post/Energy_in_breaking_bonds_ATP_and_ADP/attachment/5e525ed5cfe4a7bbe5617e1e/AS%3A861699309584385%401582456533095/download/IMG_4426.jpg)